The chemical element hydrogen has the symbol H and the atomic number 1. The lightest element is hydrogen. Under normal circumstances, hydrogen is a gas composed of diatomic molecules with the formula H2. It’s odorless, colorless, non-toxic, and extremely flammable. Hydrogen is the most abundant chemical element in the universe, accounting for around 75% of all ordinary stuff. The Sun, for example, is mostly made up of hydrogen in its plasma state. The majority of hydrogen on the planet is found in molecule forms like water and organic substances. Each atom of the most common hydrogen isotope (symbol 1H) has one proton, one electron, and no neutrons.
Discovery of Hydrogen Peroxide
The simplest member of the peroxide family is hydrogen peroxide, which was originally discovered as a chemical substance in 1818 by French chemist J.L Thenard. H2O2 is its molecular formula. Hydrogen peroxide can be found in very low amounts in the environment. Photochemical reactions in the atmosphere around the earth produce gaseous hydrogen peroxide. It is used as a disinfectant as well as a bleaching agent. It’s acidic, with a pH of 6 to 7 at 298 degrees Fahrenheit.
Structure of Hydrogen Peroxide
- The peroxide ion (O2–2) is present in peroxide, a chemical molecule. (O–O)2– is the peroxide ion, which is made up of a single link between two oxygen atoms. It’s a powerful oxidant.
- Hydrogen peroxide is a non-planar molecule consisting of two oxygen atoms joined by a single covalent connection known as the peroxide bond.
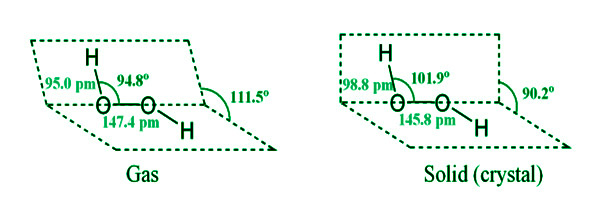
- It’s laid out like a book. Each oxygen atom is also connected to a hydrogen atom by a single link. Because lone pairs of electrons repel each other over oxygen atoms, the two bonds do not lie in the same plane. When hydrogen peroxide is in a gaseous condition, the dihedral (interplanar) angle between the two planes is 111.5o, but when it is crystalline, it is 90.2o. Intramolecular hydrogen bonding causes this to happen.
The hydrogen bond parameters in the gaseous and crystalline phases are:
Uses of Hydrogen Peroxide
Hydrogen peroxide is a fluid that can be used in a variety of ways. It works in all media, including water, air, wastewater, and soils. To improve and accelerate processes, it is sometimes coupled with other agents. The following are some of its applications:
- Pulp and paper bleaching consumes around half of the world’s hydrogen peroxide production.
- Human hair is bleached with diluted hydrogen peroxide combined with aqueous ammonia.
- New bloodstains are removed with hydrogen peroxide.
- H2O2 solution is utilized as a propellant for torpedoes and submarines, as well as an oxidant for rocket fuel.
- In aquaculture, hydrogen peroxide is utilized to decrease mortality caused by different microorganisms.
- It can be used to sterilize cosmetic brushes and sanitize toothbrushes.
- Hydrogen peroxide is a mild antiseptic that can be used for small cuts, scratches, and burns to prevent infection.
- It can also be used as a mouthwash to clear mucus and soothe minor mouth discomfort.
- Aids in the treatment of fungal infections in plants and the clearing of algae-infested ponds.
- It’s utilized in the textile sector as an antichlor to eliminate excess chlorine after bleaching.
- It is widely utilized in the production of inorganic chemicals such as sodium perborate and percarbonate, which are important components of high-quality detergents.
- It’s used to restore the color of lead paintings that have become darkened as a result of the effect of H2S in the air on lead paints.
Properties of Hydrogen Peroxide
The following are the physical and chemical properties of hydrogen peroxide:
Physical Properties of Hydrogen Peroxide
- In its anhydrous state, hydrogen peroxide is a pale blue color. Due to H– bonding, it is an odorless thick syrupy liquid.
- It has a bitter taste that causes skin blisters.
- It is water, alcohol, and ether soluble.
- Hydrogen peroxide is more viscous and denser (1.44g/cm3) than water. This is due to the fact that H2O2 molecules have stronger H– bonds than H2O molecules.
- It has a boiling temperature of 150 degrees Celsius and a freezing point of –0.89 degrees Celsius. The boiling point of hydrogen peroxide rises even higher because the intermolecular hydrogen bonding is stronger than that of water.
- Hydrogen peroxide has a little higher dipole moment (2.1D) than water (1.84D).
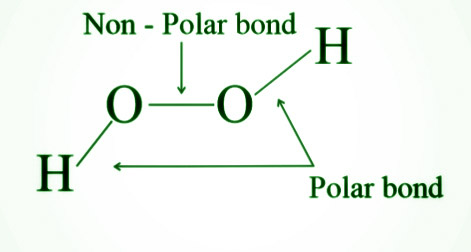
- Hydrogen peroxide has both polar and nonpolar bonds and is diamagnetic.
Chemical Properties of Hydrogen Peroxide
Because of its molecular structure, H2O2 is a one-of-a-kind material. It is made up of oxygen atoms in the oxidation state of -1, as opposed to the oxidation states of 0 or -2 in many other compounds. This indicates that, depending on the pH of its solution, this chemical can act as both an oxidizing and a reducing agent.
- Decomposition
- Decomposition by exposure to light: Exposure to light also decomposes H2O2. As a result, it’s kept in wax-lined glass or plastic vessels with stabilizers like urea.
- Auto-oxidation and auto-reduction: Hydrogen peroxide in its purest form is a highly unstable liquid. When left out for a long time or heated, it decomposes into water and oxygen.
The presence of metals such as platinum, gold, metal oxides (MnO2), or specific metal ions such as Fe2+ ions speeds up the breakdown process. Its breakdown is aided by even a rough surface.
- Acidic Nature
- H2O2 is a weak acid since it turns blue litmus red. Litmus is unaffected by its aqueous solution. H2O2 is just marginally stronger than H2O because its dissociation constant (1.55×10–12 at 293K) is slightly higher than H2O’s (1.0×10–14).
- The neutralizing reactions of hydrogen peroxide with hydroxides and carbonates demonstrate its acidic character. For example, Ba(OH)2+H2O2→BaO2+2H2O
- Since H2O2 comprises two ionizable H–atoms, it generates two types of salts: hydroperoxides (acidic salts) and peroxides (peroxides) (normal salts).
- Reducing Property-
Hydrogen peroxide acts as a reducing agent in both acidic and alkaline mediums when powerful oxidizing agents are present. The combination of H2O2 and the nascent oxygen [O] generated by the strong oxidizing agent produces molecular oxygen in all of these processes.
H2O2 + [O] (From oxidizing agent) → H2O + O2
- In acidic medium: Hydrogen peroxide loses electrons and is oxidized to O2 in an acidic environment.
H2O2(O.S=–1)→2H++O2(O.S=0)+2e–[Oxidation]
For example,
1) H2O2 turns a pink acidified potassium permanganate solution into a colorless solution.
2KMnO4+3H2SO4+5H2O2→K2SO4+2MnSO4+8H2O+5O2
2) In the presence of dilute sulphuric acid, it converts manganese dioxide to manganese sulphate.
MnO2(aq)+2H+(aq)+H2O2→Mn2+(aq)+2H2O(l)+O2(g)
- In alkaline medium: Hydrogen peroxide is converted to O2 in an alkaline media.
H2O2+2OH–→2H2O+O2+2e
For example,
1) Ferric salts are converted to ferrous salts.
2Fe3+(aq)+H2O2(aq)+2OH–(aq)→2Fe2+(aq)+O2(g)+2H2O(l)
2) In the basic media, it converts iodine to iodide ions.
I2(s)+H2O2(aq)+2OH–(aq)→2I–(aq)+2H2O(l)+O2(g)
H2O2 is a powerful oxidising agent because it readily takes electrons and is reduced in both alkaline and acidic environments.
- In an acidic medium: In the presence of an acidic media, H2O2 can receive electrons and behave as an oxidising agent. H2O2 is decomposed into H2O.
H2O2+2H++2e–→2H2O( Eo=+1.77V)
For example,
1) Acidified ferrous sulphate is converted to ferric sulphate.
2Fe2+(aq)+H2O2(aq)+2H+(aq)→2Fe3+(aq)+2H2O(l)
2) It extracts iodine from a potassium iodide solution that has been acidified.
2I–(aq)+H2O2(aq)+2H+(aq)→I2(s)+2H2O(l)
- In an alkaline medium: In an alkaline media, hydrogen peroxide can take electrons and operate as an oxidising agent.
H2O2+OH–+2e–→3OH–
For example,
1) When hydrogen peroxide oxidises manganese salts to manganese dioxide, a brownish precipitate results.
Mn2+(aq)+H2O2(aq)+2OH–(aq)→MnO2(s)+2H2O(l)
2) When chromium sulphate is oxidized by hydrogen peroxide in an alkaline media, the dark green colour changes to yellow sodium chromate.
Cr2(SO4)3+3H2O2+10NaOH→2Na2CrO4+3Na2SO4+8H2O
- In neutral medium: In a neutral media, hydrogen peroxide oxidises a wide range of substances. For example,
1) It oxidizes sulphites to sulphates.
SO2–3+H2O2→SO2–4+H2O
2) It converts nitrites to nitrates by oxidising them.
NO–2+H2O2→NO–3+H2O
Preparation of Hydrogen Peroxide
Laboratory preparation
- From sodium peroxide (Merck’s Process): Sodium peroxide is introduced in small amounts to a weak solution of sulphuric acid (20%) surrounded by ice and constantly stirred in this procedure. When the solution is cooled further, crystals of Na2SO4. 10H2O form, which can be filtered out. The solution is a 30 percent hydrogen peroxide aqueous solution.
Na2O2+H2SO4→Na2SO4+H2O2
- From barium peroxide: A hydrated barium peroxide (BaO2.8H2O) paste made in ice-cold water is treated with a 20% ice-cold sulphuric acid solution. Filtration is used to remove the BaSO4 precipitate, which is white in color. About 5% H2O2 remains in the solution.
BaO2⋅8H2O+H2SO4→BaSO4(White ppt)+H2O2+8H2O
This method is inefficient because barium sulfate generates a protective coating around H2O2 that inhibits it from continuing the chemical reaction. The Ba2+ ions in the solution slowly decompose hydrogen peroxide. As a result, the solution cannot be stored for an extended period of time. Phosphoric acid, rather than sulphuric acid, is used to test this. The barium phosphate that forms is entirely precipitated, and there is no threat of hydrogen peroxide breakdown in the absence of Ba2+ ions.
3BaO2⋅8H2O+2H3PO4→Ba3(PO4)2(ppt)+24H2O+3H2O2
Industrial Preparation
- By the electrolysis of a sulphuric acid solution: In a cell, a 50 % sulphuric acid solution is electrolyzed. Peroxodisulfuric acid is generated at the anode, and hydrogen is released at the cathode as a result.
H2SO4→HSO–4+H+
At anode: HSO–4→H2S2O8 (peroxide sulphuric acid) +2e–
At cathode: 2H++2e–→H2
The cell’s peroxide sulfuric acid is removed and decomposed with water to produce hydrogen peroxide.
H2S2O8+2H2O→2H2SO4+H2O2
Sulphuric acid with a high boiling point does not distill, although hydrogen peroxide does. When a mixture of ammonium sulfate and sulphuric acid in equal proportions is electrolyzed, the output of hydrogen peroxide can be increased.
The hydrogen peroxide is obtained by distilling the ammonium peroxide sulphate produced at the anode with water.
(NH4)2S2O8+2H2O→2NH4HSO4(Ammoniumhydrogensulphate)+H2O2
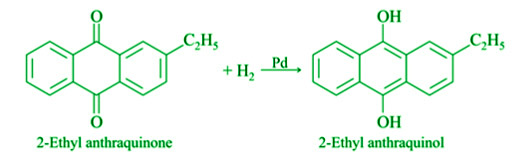
- From 2-Ethyl anthraquinone: Hydrogen gas is transported through 2-ethyl anthraquinone dissolved in benzene in the presence of a palladium catalyst. It’s broken down into 2ethyl anthraquinol. A mixture of 2ethyl anthraquinol, benzene, and cyclohexanol is then circulated through the air. Hydrogen peroxide is created when it is oxidised back to 2ethyl anthraquinone.
- By oxidation of isopropyl alcohol: When a little amount of hydrogen peroxide is combined with isopropyl alcohol, it works as an initiator. At around 340K, and with a little pressure, oxygen is transferred through the solution. Acetone and hydrogen peroxide are produced as a result of the oxidation reaction.
CH3CHOHCH3(Isopropyl alcohol)+O2→CH3COCH3(Acetone)+H2O2
Sample Questions
Question 1: Is it safe to use hydrogen peroxide?
Answer:
Most people are safe when they utilise hydrogen peroxide correctly. However, if a person takes the substance too frequently or in a high dosage, it might be dangerous. To avoid irritation, it’s critical to use a concentration of no more than 3% and to use it sparingly. Because there is a risk of swallowing hydrogen peroxide, children should avoid handling it.
Question 2: Does hydrogen peroxide kill germs?
Answer:
When hydrogen peroxide is allowed to stay on surfaces for at least 10 minutes at room temperature, it is most effective. Hydrogen peroxide can deactivate a wide range of microorganisms, including viruses, bacteria, fungus, and spores, and acts as a disinfectant by eliminating critical components of germ cells.
Hydrogen peroxide is a good option to use on inanimate surfaces like metal, glass, and plastics when it comes to reducing germs in your home and containing the spread of Covid19. According to the Centers for Disease Control and Prevention (CDC) (CDC).
Question 3: Is hydrogen peroxide stronger than bleach?
Answer:
When we talk about bleach, we’re talking about chlorine bleach, which is made up of sodium hypochlorite. Bleach, like hydrogen peroxide, produces nascent oxygen, which is a bleaching agent. Despite the fact that bleach is more powerful than hydrogen peroxide, it is a very dangerous toxin. It requires careful dilution for safe use, and only cold water should be used. Hydrogen peroxide, on the other hand, has a lower environmental impact and is effective in treating wastewater and disinfecting substances.
Question 4: Will rinsing with peroxide whiten teeth?
Answer:
Gargling with hydrogen peroxide may help to relieve a sore throat, disinfect the mouth, and whiten the teeth. However, it should be diluted. If swallowed, a higher concentration of hydrogen peroxide causes internal organ damage as well as excessive bleeding. While rinsing with the diluted solution, prevent ingestion. To develop whiter teeth with merely gargling or rinsing, though, you’ll need to do it for a long time.
Question 5: What is Merck’s process?
Answer:
In this technique, sodium peroxide is added in minute amounts to a weak sulphuric acid (20%) solution covered by ice and regularly stirred. Crystals of Na2SO4. 10H2O occur as the solution is cooled further, and they can be filtered out. A 30 percent hydrogen peroxide aqueous solution is used.
Na2O2+H2SO4→Na2SO4+H2O2
Question 6: What is the difference between water and hydrogen peroxide?
Answer:
Water and hydrogen peroxide have different physical properties according to the extent of hydrogen bonding. Peroxide has a higher hydrogen bonding strength than water because it has one more oxygen atom than water, allowing for greater hydrogen bonding.
Because of the strain in the O–O bond in hydrogen peroxide, the chemical characteristics of water and hydrogen peroxide differ. The lone pairs on two oxygen atoms in hydrogen peroxide cause a lot of strain, weakening the O–O bond and allowing it to breakdown quickly into water and oxygen. With the exception of the water molecule, this is not the case. It doesn’t have any O–O bond tension.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...