Isomerism in Coordination Compounds as the name suggests explores the concept of Isomerism in Coordination Compounds i.e., generally compounds formed by d-block elements. Isomerism is the phenomenon of exhibiting different molecular structures by the compounds with same molecular formula. The phenomenon of isomerism is quite significant in hydrocarbons however it is of no less importance in coordination compounds. Coordination Compounds are those chemical compounds in which a group of anions is attached to a central metal atom via covalent bonds. Coordinate compounds are also called coordinate complexes. The coordinate compounds having the same molecular formula but different arrangements of ligands are called isomers of coordination compounds and the phenomenon exhibited is called Isomerism in Coordination Compounds. In this article, we will learn about different types of isomerism exhibited by Coordination Compounds in detail.
What are Coordination Compounds?
Coordination Compounds are chemical compounds in which the central metal atom or ion is attached to the number of oppositely charged or neutral atoms more than its normal valency. The coordination compounds are formed by the combination of two or more simple and stable compounds that retain their identities. The central atom is usually a transition metal due to its nature of exhibiting variable valency properties as they have the presence of incompletely filled d-orbitals.
The Central Metal Atom has two types of valencies namely primary valency and secondary valency. The primary valency tells the oxidation number and the secondary valency tells about the coordination number. The d-block transition element to which negative or neutral molecules are attached is called the central atom and the neutral atom or the anion attached to the central atom is called ligand. The central atom and the ligand are written inside a square bracket and are often called Coordination Complexes. Coordination Compounds differ from Double Salt in the manner that double salt dissociates on dissolution while Coordination Compounds don’t.
Coordination Compound Examples
Let’s understand more about coordination compounds with the help of a few examples:
In [Co(NH3)6]Cl3 the central atom is Cobalt(Co), to which six neutral molecules of NH3 are attached which is called a ligand. These are attached to Co with primary valency and hence written inside the square bracket and the Cl3 which is written outside is the counter ion attached to the coordination complex that consists of [Co(NH3)6]3+ via secondary valency.
Learn More,
Isomerism in Coordination Compounds
The phenomenon of exhibiting different arrangements of ligands by the coordination compounds with the same molecular formula is called Isomerism in Coordination Compounds. The Coordination Compounds exhibiting isomerism are called isomers. There are two types of isomerism by coordination compounds
- Structural Isomerism
- Stereoisomerism
Let’s learn them in detail.
Structural Isomerism
The property of coordination compounds having the same number of atoms of each type but showing different bonding patterns is called Structural Isomerism. The Coordination Compounds that have the same number of atoms of each type but show different bonding patterns are called structural isomers. The different types of structural isomerism are:
- Ionization Isomerism
- Hydrate or Solvate Isomerism
- Coordination Isomerism
- Linkage Isomerism
Let’s discuss these types in detail as follows.
Ionization Isomerism
Ionization Isomerism takes place when a ligand that is bound to the central atom exchanges its position with the counter ion which is outside the Coordination Complex. The Coordinate Compounds formed by such exchange of place of the ligand with the counter ion are called Isomers. For Example, [Co(NH3)5Cl]SO4 and [Co(NH3)5(SO4)]Cl are ionization isomers. This is because the ligand Cl which is a part of the Coordination Complex in [Co(NH3)5Cl]SO4 exchanges place with the Counter ion SO4 and hence now Cl becomes the counter ion and SO4 becomes the ligand.
Solvate Isomerism
Solvate Isomerism is a special type of Ionization Isomerism in which the ligand is replaced by a solvent molecule and if the solvent molecule is water then it is called Hydrate Isomerism. For Example, [Cr(H2O)6]Cl3 is a solvate isomer. It is violet in colour. In this, we see that the H2O molecule is directly attached to the central atom Cr. [CrCl(H2O)5]Cl2.H2O is a grey-green coloured hydrate isomer. However, in this case, we see that water molecule is present inside and outside the square bracket. The water molecule inside the square bracket is directly bonded to the central atom while that outside the bracket isn’t attached to the central atom and is called Water of Crystallization.
Water of Crystallization refers to the number of molecules of water attached to the compound but not part of the Coordination Complex. It is usually shown attached to the molecule by a dot(.)
Coordination Isomerism
Coordination Isomerism is exhibited when there is an exchange of ligands between a cationic and an anionic complex bonded together. In this, there is a Cationic Coordinate and an Anionic Coordinate Complex which are bonded together and there is an exchange of one or more ligands between them. For Example are [Co(NH3)6][Cr(C2O4)3] and [Co(C2O4)3][Cr(NH3)6] are Coordinate Isomers. In [Co(NH3)6][Cr(C2O4)3] (NH3)6 is attached to the Co atom and (C2O4)3 is attached to the Cr atom while in [Co(C2O4)3][Cr(NH3)6], Co is attached to C2O4 and Cr is attached to the (NH3)6.
Linkage Isomerism
Linkage Isomerism occurs when the ligand coordinates in more than one way. Such ligands that coordinate in more than one way are called ambidentate ligands. Examples of ambidentate ligands include SCN-/ NCS- and NO2– and ONO–. Examples of linkage isomerism include [Co(NH3)5(NO2)]Cl2 and [Co(NH3)5(ONO)]Cl2. In [Co(NH3)5(NO2)]Cl2, Co is attached to NO2 and called Nitro isomer while in [Co(NH3)5(ONO)]Cl2, the central atom Co is attached to the ONO and called Nitrito isomer.
Learn more about Isomerism.
Stereoisomerism
Stereoisomerism refers to the phenomenon of exhibiting different 3D spatial arrangements of bonds but having the same molecular formula. The compounds exhibiting stereoisomerism are called Stereoisomers. Stereoisomers have even the same set of bonds but they differ in arrangement. There are two types of Stereoisomerism that are:
- Geometrical Isomerism
- Optical Isomerism
These two types are discussed in detail as follows.
Geometrical Isomerism
There are two types of arrangements possible under Geometrical Isomerism. These are called cis-isomers and trans-isomers. In cis-isomers, the two similar bonds are on the same side of the central atom while in trans-isomer the similar bonds are on the opposite side of the central atom. The image inserted below will give a clear idea of cis and trans-Geometrical Isomerism.
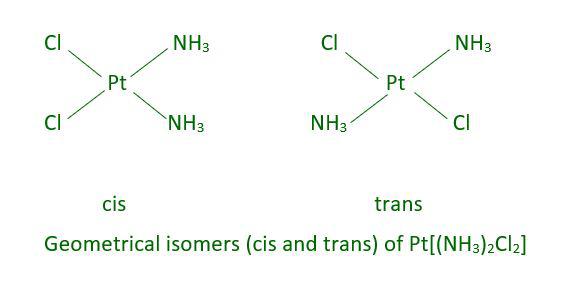
Geometrical isomerism is shown by square planar and octahedral-shaped molecules. Tetrahedral-shaped molecules don’t exhibit cis and trans isomerism. Square-shaped molecules of type MX2L2 exhibit cis and trans isomers. An example of this type is mentioned above i.e. Pt[(NH3)2Cl2]. In this case, the ligands X and L are unidentate. A square planar molecule of type MABXL where M is the central atom and A, B, X, and L are ligands all different then it will exhibit three isomers out of which two will be cis type and one will be trans.
In the case of an Octahedral Coordination Complex of type MA2B4 the cis and trans isomerism is shown below in the attached figure. In this case, the ligand which is two in number is arranged in such a manner as to exhibit cis and trans isomerism.
![Geometrical Isomers of [Co(NH3)4Cl2]](https://media.geeksforgeeks.org/wp-content/uploads/20220131122534/g2.JPG)
In the case of a Coordination Complex where the central atom is attached to a didentate ligand i.e. the ligand which has two electron donor atoms, the geometrical isomers are shown below in the picture. The compound is of the type [MX2(L-L)2] where M is the central metal atom, X is the unidentate ligand and L is the didentate ligand. In the picture attached below Co is the central atom, Cl is the unidentate ligand and en is the bidentate ligand which stands for Ethylenediamine whose molecular formula is C2H4(NH2)2.
![Geometrical Isomer of [CoCl2(en)2]](https://media.geeksforgeeks.org/wp-content/uploads/20220131123744/g3.JPG)
In the case of an Octahedral Complex of type MA3B3, there two types of isomers formed known as Facial or fac-isomer and Meridional or mer-isomers. For Example, the fac-isomer and mer-isomer for [Co(NH3)3(NO2 )3] are given below. The facial (fac) isomer is formed when three donor atoms of the same ligand occupy adjacent positions at the corners of an octahedral face. The meridional (mer) isomer is obtained when the positions are centred on the octahedron’s meridian.
Learn more about Geometric and Optical Isomerism in Coordination Compounds.
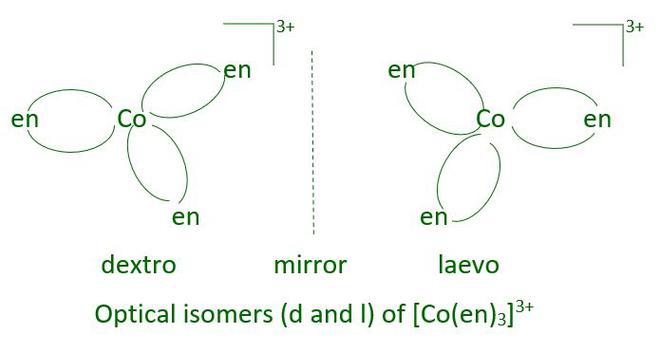
Optical Isomerism
Optical Isomerism is the phenomenon exhibited by molecules when they have similar bonds, and different arrangements such that their mirror images are non superimposable. The mirror images of molecules that can’t be superimposed on each other are called Optical Isomers. Optical Isomers are also called Enantiomers. These molecules rotate the plane of Polarized light in a Polarimeter. Depending upon the direction of rotation they are classified as Dextrorotatory and Laevorotatory. The Dextrorotatory rotates the plane of light clockwise or in the right-hand direction while Laevorotatory rotates the light rotates the plane of light in Anticlockwise or left-hand direction.
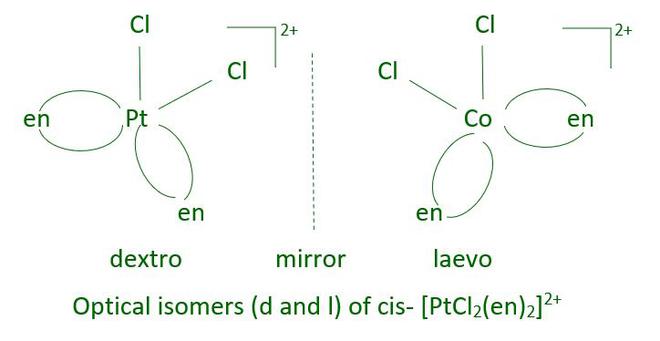
The Optical Isomerism in Coordination Compounds is exhibited by Octahedral and Tetrahedral Coordinate Complexes but not by Square Planar. Hence, we see that Octahedral Coordinate Complexes exhibit both Geometrical and Optical Isomerism. In octahedral complexes containing didentate ligands, optical isomerism is common.
In a coordination entity of the type [PtCl2 (en)2 ]2+, only the cis-isomer shows optical activity.
Also, Read
Isomerism in Coordination Compounds Examples
Example 1: Draw all geometrical isomers of [Fe(NH3 )2 (CN)4 ]–.
Solution:
![geometrical isomers of [Fe(NH3 )2 (CN)4 ]–](https://media.geeksforgeeks.org/wp-content/uploads/20220131185544/g8.JPG)
Example 2: Indicate the types of Isomerism exhibited by the following complexes
- [Co(en)3 ]Cl3
- [Co(NH3 )5 (NO2 )](NO3 )2
- [Pt(NH3 )(H2O)Cl2 ]
Solution:
- [Co(en)3 ]Cl3 —-> Optical isomerism
- [Co(NH3 )5 (NO2 )](NO3 )2 —-> Optical isomerism, Linkage isomerism, Ionization isomerism
- [Pt(NH3 )(H2O)Cl2 ] —-> Geometrical (cis-, trans-) isomerism
Example 3: How many Geometrical Isomers will be possible in [Co(NH3)3(Cl)3]?
Solution:
Two geometrical isomer is possible for [Co(NH3)3(Cl)3]–
![geometrical isomer for [Co(NH3)3(Cl)3]-](https://media.geeksforgeeks.org/wp-content/uploads/20220131190713/g9.JPG)
Example 4: What are Ligands in Coordination Compounds?
Solution:
Ligands are ions or molecules that are bound to the central atom/ion of the coordination entity. Simple ions like Cl–, small molecules like H2O or NH3, larger molecules like H2NCH2CH2NH2 or N(CH2CH2NH2)3 , and even macromolecules like proteins can fall into this category.
Example 5: Draw the geometrical isomers of [Pt(NH3)(Br)(Cl)(Py)].
Solution:
![Geometrical Isomer of [Pt(NH3)(Br)(Cl)(Py)]](https://media.geeksforgeeks.org/wp-content/uploads/20230717164458/g10-660x143.jpeg)
FAQs on Isomerism in Coordination Compounds
1. What is Coordination Compound?
Coordination Compounds are chemical compounds in which the central atom is a transition metal and negative or neutral ligands are attached more than the normal valency of the central atom.
2. What are Isomers?
Isomers are chemical compounds that have the same molecular formula but different molecular structures.
3. What are the Two different types of Isomers?
The two different types of isomers are Structural Isomers and Stereo Isomers.
4. What are Cis and Trans Isomers?
Cis and Trans isomers are the types of Stereoisomers. In Cis-isomers the two similar bonds with the central atom are on the same side of the central atom while in trans-isomer the two similar bonds are on the different sides of the central atom.
5. What are Ligands?
Ligands are the negative or neutral atoms attached to the central atom in a number more than the usual valency of the central atom.
6. What is Coordination Complex?
The Central Atom together with ligands are called the Coordination Complex.
7. What is Water of Crystallization?
The number of water molecules attached to the crystal of a molecule is called Water of Crystallization. For Example in CuSO4.5H2O, the water number of water molecules attached to the crystal is 5, hence the Water of Crystallization for Copper Sulfate Pentahydrate is 5.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...