Some Important Compounds of Sodium
Last Updated :
29 Nov, 2021
Sodium is a soft metal, it is the eleventh element in the periodic table. It is represented by the Na symbol and the atomic number of sodium is 11 it belongs to the family of s-block elements in the periodic table.
Sodium is the sixth most abundant element. Its amount in the earth’s crust is nearly 2.8% by mass. Sodium occurs in compound forms, one of the most prominent sources of sodium is sodium chloride also known as common salt which is available in seawater in large quantities.
It is one of the alkaline metals. Sodium is one of the most abundant elements that are dissolved in seawater. Sodium is a necessary ingredient for human beings but if consumed in excess then it can damage kidneys and it also causes problems of high blood pressure.
Properties of Sodium are:
- Its appearance is silvery white.
- Sodium has a low melting point of 97.8ο Celsius.
- It is an extremely reactive element and that’s why it is never found in a free state.
- Sodium is able to conduct heat and electricity.
- Sodium is a very light element even lighter than water.
- At room temperature sodium can be easily cut with a knife.
- Due to its reactive nature, it is stored under anhydrous mineral oil.
- At low temperatures, sodium is brittle in nature.
Uses of Sodium are:
- Sodium is used by every human being in the form of sodium chloride also known as common salt.
- It is used to make sodium vapour lamps.
- It is also used in the synthesis of polymers like nylon.
- One of the most common uses of sodium is in making gasoline additives.
- Sodium is also used in the formation of sodium cyanide.
- Many dyes and perfumes are also made using some amount of sodium.
- Sodium is also used for the purpose of purification of hydrocarbons.
Some important Compounds of Sodium
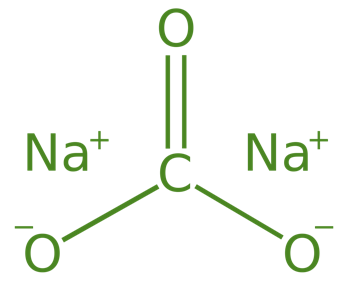
Sodium Carbonate (Na2CO3.10H2O)
Sodium Carbonate is also known as Washing Soda, its formula is Na2CO3.10H2O and its chemical name is sodium carbonate decahydrate. Sodium Carbonate is mainly prepared by the Solvay process.
Solvay process is a 4 step process.
- It starts with the reaction of ammonia, water and carbon dioxide which results in the formation of ammonium carbonate.
2NH3 + H2O + CO2 → (NH4)2CO3
- Now ammonium carbonate is again made to react with water and carbon dioxide to form ammonium hydrogen carbonate.
(NH4)2CO3 + H2O + CO2 → 2NH4HCO3
- Now ammonium hydrogen carbonate is mixed with sodium chloride to get ammonium chloride and sodium hydrogen carbonate.
NH4HCO3 + NaCl → NH4Cl + NaHCO3
- Last step is to heat sodium hydrogen carbonate crystals to get sodium carbonate.
2NaHCO3 → Na2CO3 + CO2 + H2O
Properties of Sodium Carbonate
- It occurs in the form of a white crystalline solid.
- It is called decahydrate because it has 10H2O attached to it which can be removed by heating it.
- Sodium carbonate can be easily dissolved in water.
- If sodium carbonate is heated above 100ο C then it will change to a white coloured powder known as soda ash.
- It is alkaline in nature.
Uses of Sodium Carbonate
- Sodium carbonate is used for the softening of hard water.
- It is used in the soap and paint industries for the manufacturing of soaps, detergents and paints.
- Used in glass and textiles industries as well.
- It is also used in the manufacturing process of glass.
- The Paper industry also uses sodium carbonate in the papermaking process.
Sodium Chloride
Sodium chloride is also known as common salt, its formula is NaCl. It is mainly found in seawater. It can be easily obtained from seawater by the process of evaporation.
Solar evaporation is the process that is used to produce sodium chloride commercially from seawater, it is found that every year India produces around fifty lakh tons of sodium chloride for multiple purposes.
Sodium chloride obtained after the evaporation of seawater is not pure and different processes are used to make it pure. Sodium chloride is also found in rock forms under the earth surface which is formed by a decomposition process that took millions of years.
Properties of Sodium Chloride
- Sodium chloride is in the form of colourless crystals.
- It is easily soluble in water.
- It has a high melting point of 801ο Celsius.
- Sodium chloride solution can easily conduct electricity.
- Sodium chloride has a cubic closely packed structure.
- Sodium chloride does not have any odour means it is odourless but it has a salty taste.
- Sodium chloride is insoluble in oil.
Uses of Sodium Chloride
- Used as an ingredient for making food.
- It is also used as a preservative for example in the preservation of meat.
- Used in the preparation of caustic soda and washing soda.
- Liquid sodium is used as a coolant in nuclear reactors.
- Used by the body to transmit nerve signals.
- It is used to remove grease stains.
- It is also used in many eyes drops to treat eye redness and some other common eye problems.
- In case of low blood pressure, sodium chloride with water is given to maintain blood pressure.
Sodium Hydroxide
Sodium Hydroxide is also known as Caustic Soda and its formula is NaOH. It is prepared by Sodium chloride electrolysis which is performed in a special type of cell called the Castner-Kellner cell. Take mercury as cathode and carbon rode as an anode, sodium chloride solution is now electrolyzed. Formation of sodium amalgam takes place at cathode and chlorine gas is released at anode. The process can be easily understood by the following reactions at anode and cathode.
At Cathode: Na+ + e– → Sodium amalgam
At Anode : Cl– → 1/2Cl2 + e–
Now sodium amalgam is treated with water to get sodium hydroxide or caustic soda along with hydrogen gas and mercury.
2Na-Amalgam + 2H2O → 2NaOH + 2Hg + H2
Properties of Sodium Hydroxide
- It has a high boiling point of 1390ο Celsius.
- Can be easily dissolved in water or alcohol.
- Its appearance is white solid.
- Sodium hydroxide is insoluble in ether.
- The melting point of sodium hydroxide is 318.4ο Celsius.
- It is toxic in nature.
Uses of Sodium Hydroxide
- Mainly used in the soap manufacturing process.
- It can be used to clean drains in their concentrated form.
- Used to refine petroleum in the petroleum industry.
- Sodium hydroxide plays an important role in the Bayer process which is used to produce aluminium.
- Can be used for the extraction of zinc and plating of tin.
Sample Questions
Question 1: What is the nature of Sodium Hydroxide?
Answer:
Sodium hydroxide is basic in nature as it releases hydroxide ions in water. The basic nature of sodium hydroxide can be tested by reacting it with an acid say HCl because when acid and bases are reacted they form salt and water.
NaOH + HCl → NaCl + H2O
Now it’s clear that NaOH is basic in nature.
Question 2: How de-icing of slippery roads and bridges is done using NaCl?
Answer:
NaCl or common salt is used in the de-icing of slippery roads as NaCl can decrease the freezing point of water so it will become difficult the process of ice forming but simply salt cannot do much, water must also be present hence salt solution which is also known as brine is needed for de-icing of slippery roads.
Question 3: How NH3 can be recovered used in the Solvay process to make Sodium carbonate?
Answer:
NH3 can be recovered by treating NH4Cl (which is formed in the third step) with Calcium hydroxide.
2NH4Cl + Ca(OH)2 → 2NH3 + CaCl2 + H2O
Question 4: How does Baking Soda make cakes and pastries soft and fluffy?
Answer:
Baking soda also known as Sodium Hydrogencarbonate carbonate (NaHCO3) is mainly used to make cakes and pastries fluffy and soft. It makes them light as it releases carbon dioxide to make bubbles when heated.
Questions 5: How sodium is biologically important?
Answer:
Sodium ions are present on the surface of cells which helps in regulating water and other sugar compounds in cells, sodium ions also help in the transmission of nerve signals to different body parts.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...